WARNING:
Proper Cleaning Methods of the OKLand® PREP.
It is important to understand that this PREP is very valuable in its function. However, it can be vulnerable if cleaned improperly.
Here is a more detailed and focused page to explain the best practices for cleaning and maintaining the OKLand® PREP.
Keep your PREP Safe and Reliable!
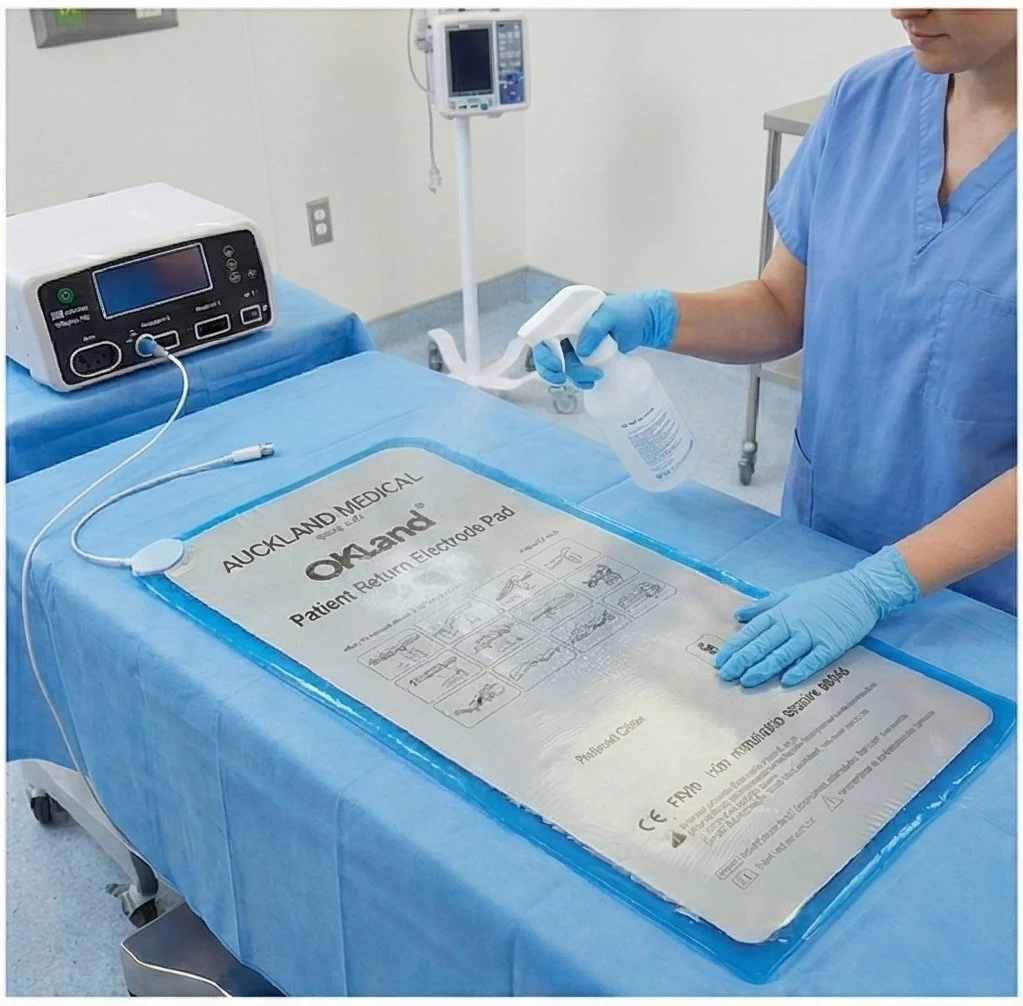
To clean and disinfect the device, the following guidelines should be followed, which are included in the Instructions for Use (IFU).
NOTE:
Hydrogen Peroxide, Isopropyl Alcohol or High % Bleach products should NOT be used.
1. The pad and the cable should be cleaned if they get contaminated and should be cleaned at least once each day.
2. Use clear water to clean any fluids, such as blood, from the device. Then clean the device with any of the following solutions:
Mild bleach solution (10:1),
Glutaradehyde,
Ethanol (recommended concentration, 70% -75%),
o-Phenylphenol,
o-Benzyl-p-chlorophenol or,
p-Tertiary amylphenol.
3. For special diseases like Tetanus, AIDS, and Hepatitis B, use of a special protective film is strongly recommended to cover the pad. After use, throw away the protective film.
4. The use of iodine cleaning agent may discolor the pad, but will not influence the safety and effectiveness of the device.
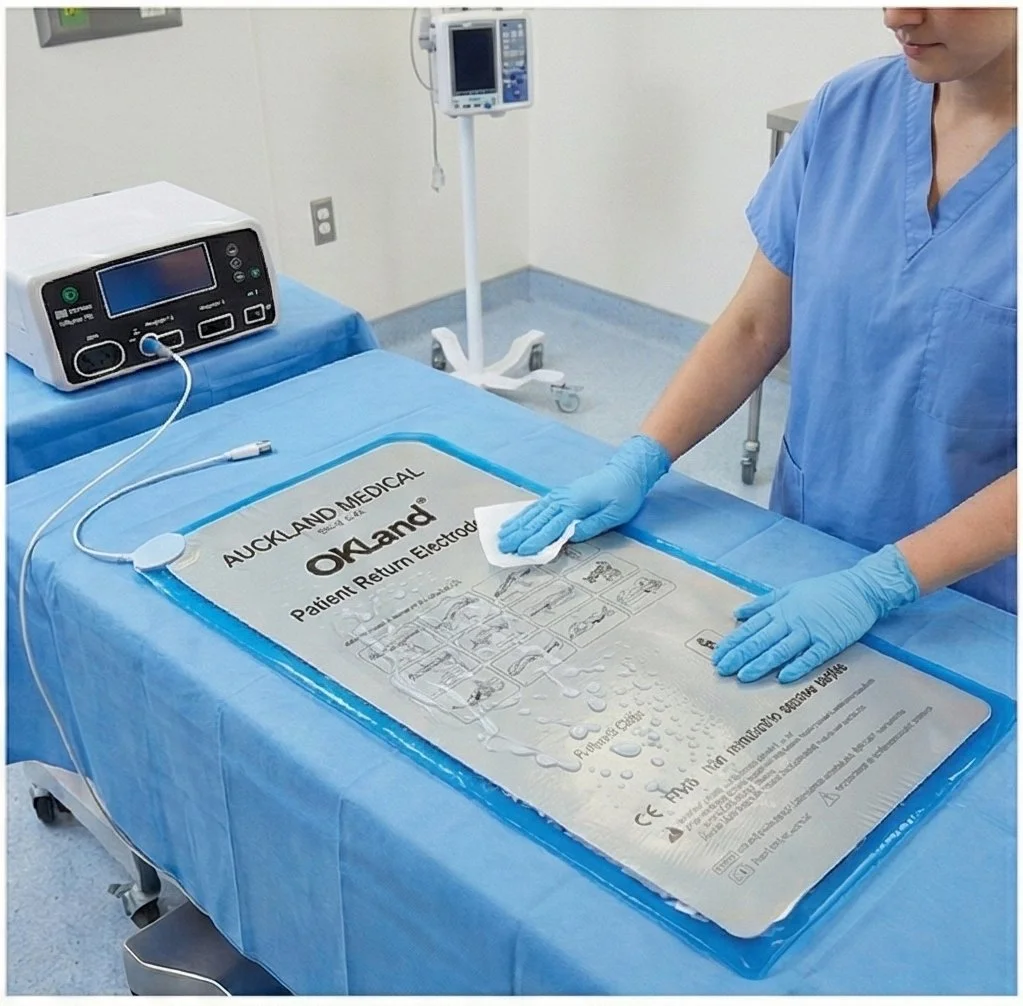
5. Thoroughly rinse the device with clear water to remove any residue of the cleaning solutions.
7. Do not use ultraviolet rays, gas sterilization, autoclaving, washing machine, dryer or pro-longed soaking in disinfectants.
8. Avoid cleaning solutions or rinse water to come in contact with metal connectors.
9. Dry the device prior to next use.
STEP 1. Pre-Clean
Clear water only to remove visible blood or bodily fluids.
STEP 2. Disinfect
Ethanol (70–75%), Glutaraldehyde, o-Phenylphenol, o-Benzyl-p-chlorophenol, p-Tertiary amylphenol
STEP 3. Post-Clean
Rinse thoroughly with clear water; dry completely before next use.
STEP 4. Special Cases
Use disposable protective film for HIV/AIDS, Hepatitis B, Tetanus; discard after use.
APPROVED CLEANING & DISINFECTION METHOD
NOT APPROVED - DO NOT USE
Bleach products / wipes (Sodium hypochlorite)
Corrosive; can damage pad materials
Isopropyl alcohol products
Can cause drying, cracking, and material degradation
Hydrogen peroxide disinfectants
Explicitly prohibited
Other strong or undiluted disinfectants
Not material-compatible
UV, gas sterilization, autoclave, washer/dryer
Not permitted
Prolonged soaking in disinfectants
Can cause device damage
Contact with metal connectors
Risk of corrosion
-
The FDA announced a recall for a popular reusable PREP and that manufacturer has discontinued the entire product line.
-
A deliberate half-inch thickness to support uniform contact with the patient while providing additional comfort. Multiple size configurations are available from 16”x16” to 47”x20”. Heavy duty profile for extended use and durability. It also works well through a sheet avoiding the need for direct skin contact.
-
Because The OKLand® PREP is non-adhesive and can cover a large surface area, it can be considered the only viable option where adhesives are undesirable (e.g., burns, grafts, fragile skin).
It is also thicker to provide additional comfort to the patient. -
Our largest size comes in a Single and Dual Cord set-up , measures at 47” x 20” x 0.5” and weights approximately 24 lbs.
-
The OKLand® PREP is compatible with the most common ESU’s via custom cable options. We have a compatibility chart to help determine the best cable for your ESU.