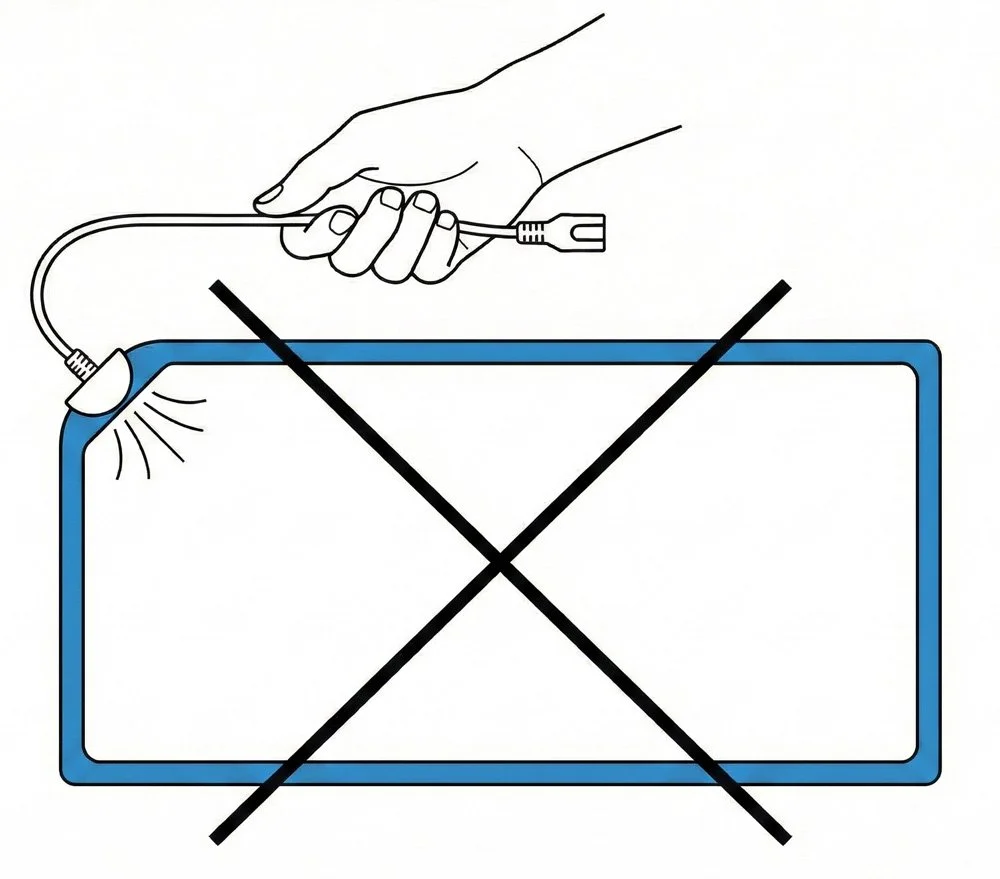
INCORRECT HANDLING
WARNING:
Proper Handling & Storage of the OKLand® PREP.
It is important to understand that this PREP is very valuable in its function. However, it can be vulnerable if handled or stored improperly.
Here is a more detailed and focused page to explain the best practices for Handling, Transporting & Storing the OKLand® PREP.
Keep your PREP Safe and Reliable!
For proper storage and transportation of this device, the following guidelines should be followed, which are included in the
Manufacturer’s Instructions For Use (IFU).
CORRECT HANDLING, TRANSPORTATION & STORAGE METHODS
The integrated pigtail lead and female receptacle are functional components of the Patient Return Electrode Pad (PREP).
Improper handling, transport, or storage may damage the lead or receptacle and result in delays or loss of function.
FROM THE MANUFACTURER’S IFU (Storage and Transportation):
1. The device should be stored in a well-ventilated, dry room without corrosive gases, and kept away from direct sunlight.
2. Keep the device in a flat position while in storage.
3. Handle the device with extra care, never tear the edges or over-stretch the pads.
4. Keep the device away from hard and sharp objects and edges.
5. Do not pull, lift, or carry by the cable. This may cause electrical failure.
6. When transporting the pads, special care is recommended. If possible, use carts
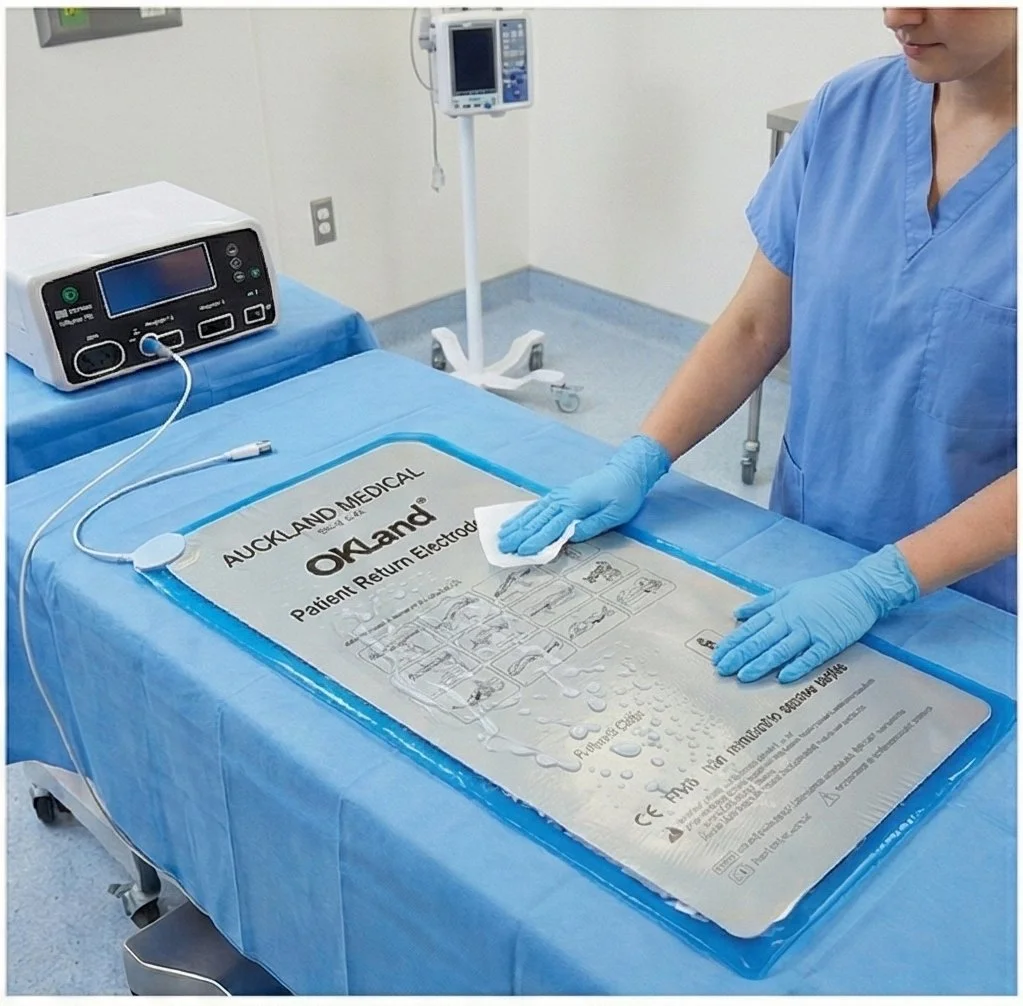
STEP 1. Pre-Clean
Clear water only to remove visible blood or bodily fluids.
STEP 2. Disinfect
Ethanol (70–75%), Glutaraldehyde, o-Phenylphenol, o-Benzyl-p-chlorophenol, p-Tertiary amylphenol
STEP 3. Post-Clean
Rinse thoroughly with clear water; dry completely before next use.
STEP 4. Special Cases
Use disposable protective film for HIV/AIDS, Hepatitis B, Tetanus; discard after use.
APPROVED CLEANING & DISINFECTION METHOD
NOT APPROVED - DO NOT USE
Bleach products / wipes (Sodium hypochlorite)
Corrosive; can damage pad materials
Isopropyl alcohol products
Can cause drying, cracking, and material degradation
Hydrogen peroxide disinfectants
Explicitly prohibited
Other strong or undiluted disinfectants
Not material-compatible
UV, gas sterilization, autoclave, washer/dryer
Not permitted
Prolonged soaking in disinfectants
Can cause device damage
Contact with metal connectors
Risk of corrosion
-
The FDA announced a recall for a popular reusable PREP and that manufacturer has discontinued the entire product line.
-
A deliberate half-inch thickness to support uniform contact with the patient while providing additional comfort. Multiple size configurations are available from 16”x16” to 47”x20”. Heavy duty profile for extended use and durability. It also works well through a sheet avoiding the need for direct skin contact.
-
Because The OKLand® PREP is non-adhesive and can cover a large surface area, it can be considered the only viable option where adhesives are undesirable (e.g., burns, grafts, fragile skin).
It is also thicker to provide additional comfort to the patient. -
Our largest size comes in a Single and Dual Cord set-up , measures at 47” x 20” x 0.5” and weights approximately 24 lbs.
-
The OKLand® PREP is compatible with the most common ESU’s via custom cable options. We have a compatibility chart to help determine the best cable for your ESU.
-
See our Cleaning Page and our Transportation & Storage Page for best practices for a long life span for your PRE Pad.